The race to produce higher performing batteries

Last week battery startup SES announced it had developed the world’s largest lithium metal cell. The cell itself is still a prototype, but the company expects to be producing them commercially for use in EVs by 2025. Off the back of this announcement, I want to talk about batteries using lithium metal anodes, and the differences between SES and its peers QuantumScape and Solid Power.
Lithium metal anodes are sought after in the battery industry, because they promise to create lightweight cells enabling EVs with longer driving ranges. Not only are companies trying to improve performance, they also are trying to make batteries safer and with a lower price tag. But they are taking different approaches to achieve this, the benefits and drawbacks of which can be understandably difficult to pick apart.
There are three key components in a lithium battery: two electrodes — the anode and the cathode — which are separated by an electrolyte. The batteries in your cell phone or EV today use a graphite for the anode and a liquid electrolyte. These are the components that most startups are working to improve, as they haven’t really changed in 30 years. Cathode development is less of a focus, as these materials have experienced steady but gradual improvements over the last decade.
Taking a step back, SES’s announcement is big news because it’s the first demonstration of a battery that could be used in an EV using a lithium metal anode with a liquid electrolyte. Using a liquid electrolyte with a lithium metal is exciting because it could almost be a ‘drop-in’ process that utilizes existing manufacturing facilities. This could lead to quick scale up and a high market penetration, while also preserving the billions of dollars of investment in current manufacturing plants.
In the past it hasn’t been possible to use lithium metal anodes with liquid electrolytes because it leads to a low-cycle life – the number of times the battery can be charged and discharged – and can cause failures leading to battery fires. SES’s secret sauce is in getting these two critical components to work together. It has achieved this by adding so much salt to its electrolyte that the properties of the liquids change, from flammable to a substance that won’t catch fire. By using a liquid SES creates good contact between all of the layers in the battery – this is essential to having a battery that can perform lots of cycles.
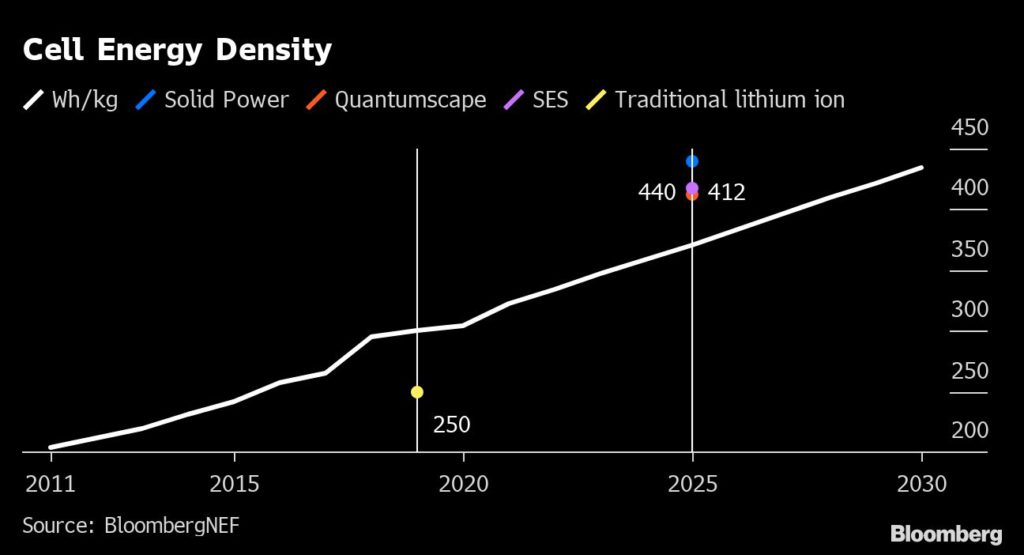
By sticking to a liquid-based electrolyte, SES’ approach differs from its peers Solid Power and QuantumScape. In contrast, Solid Power and QuantumScape have been targeting the use of lithium metal anodes in combination with a solid electrolytes, in place of liquids, to create solid-state batteries. Solid electrolytes have been the main focus of companies developing cells that use lithium metal anodes as it is easier to control the reactions between the two components, and removes the liquid electrolyte as a possible accelerant in the event of a fire.
Solid Power uses an electrolyte material called lithium sulfide, which promises to be relatively easy to integrate with existing manufacturing processes. As its cells only use solid components it is important to make sure all of the layers have good contact to ensure high performance over the lifetime of the system. Before it commercializes batteries with lithium metal anodes, Solid Power is also aiming to develop a cell with a silicon anode. But we’ll leave silicon anodes for a future column.
QuantumScape on the other hand will use a ceramic material for its cells. These will require the company to develop new manufacturing processes and equipment. However, its technology has the advantage of not using lithium metal, which is difficult to handle, during the manufacturing process. Instead, QuantumScape forms its lithium metal anode in-situ in the manufactured cell. It also adds a little gel electrolyte to help maintain contact between the cathode material and the solid electrolyte.
The eventual performance of each company’s cells promises to be fairly similar, all should be safer and with a higher energy density, but the routes to getting there are markedly different. Each company has its own unique set of challenges when it comes to scaling the production of the technology, but nothing that appears insurmountable. From an industry perspective, the more routes we have to achieving high performing cells the better, as it creates more resiliency and makes me confident safer high performance batteries are just around the corner.
(By James Frith)
{{ commodity.name }}
{{ post.title }}
{{ post.date }}




Comments