Palladium-infused ‘nano-chocolates’ a cheaper mechanism to store hydrogen

Researchers at the Deutsches Elektronen-Synchrotron (DESY) and the Universities of Cologne and Hamburg are proposing the idea of storing hydrogen in 1.2-nanometer palladium nanoparticles as a way to address the issue of costly storage systems.
In a paper published in the journal ACS Nano, the group explains that even though hydrogen holds great promise as a climate-friendly fuel that can power anything from airplanes to steel plants, storing it is still a very expensive and energy-intensive process because the gas has to be kept in pressurized tanks, at up to 700 bar, or it must be liquified, which means cooling it down to minus 253 degrees Celsius.
Since palladium is known to absorb hydrogen like a sponge, the German scientists decided to develop an alternative reservoir using the precious metal.
To ensure that the tiny particles are sufficiently sturdy, they are stabilized by a core made of iridium. In addition, they are attached to support made of graphene, an extremely thin layer of carbon.
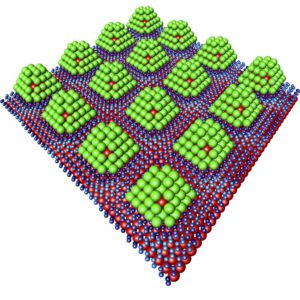
“We are able to attach the palladium particles to the graphene at intervals of just two and a half nanometers,” lead researcher Andreas Stierle said in a media statement. “This results in a regular, periodic structure.”
Using DESY’s X-ray source PETRA III, Stierle and his colleagues were able to observe what happens when the palladium particles come into contact with hydrogen: Essentially, the hydrogen sticks to the nanoparticles’ surfaces, with hardly any of it penetrating inside.
According to the researchers, the nanoparticles can be pictured as resembling chocolates: An iridium nut at the center, enveloped in a layer of palladium, rather than marzipan, and chocolate-coated on the outside by the hydrogen.
All it takes to recover the stored hydrogen is for a small amount of heat to be added; the hydrogen is rapidly released from the surface of the particles because the gas molecules don’t have to push their way out from inside the cluster.
“Next, we want to find out what storage densities can be achieved using this new method,” Stierle said.
However, he pointed out that some challenges still need to be overcome before proceeding to practical applications. As an example, he mentioned that other forms of carbon structures such as carbon sponges containing tiny pores might be a more suitable carrier than graphene.
{{ commodity.name }}
{{ post.title }}
{{ post.date }}




Comments