Graphic: How is aluminum made?
How to Use: Click the arrows on the right to see the next stage of aluminum production.
Aluminum is one of our most widely-used metals, found in everything from beer cans to airplane parts.
However, the lightweight metal doesn’t occur naturally, and producing it is a complex process.
The above infographics use data from the USGS, Aluminium Leader, and other sources to break down the three stages of aluminum production.
The three stages of aluminum production
Each year, the world produces around 390 million tonnes of bauxite rock, and 85% of it is used to make aluminum.
Bauxites are rocks composed of aluminum oxides along with other minerals and are the world’s primary source of aluminum. After mining, bauxite is refined into alumina, which is then converted into aluminum.
Therefore, aluminum typically goes from ore to metal in three stages.
Stage 1: Mining bauxite
Bauxite is typically extracted from the ground in open-pit mines, with just three countries—Australia, China, and Guinea—accounting for 72% of global mine production.
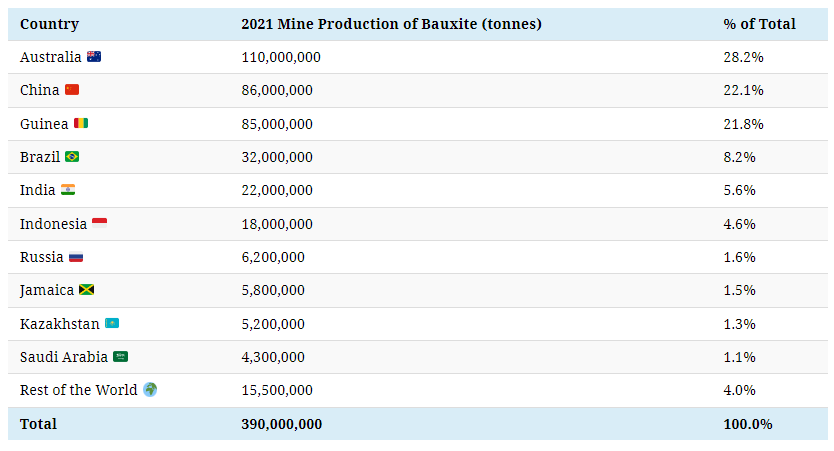
Australia is by far the largest bauxite producer, and it’s also home to the Weipa Mine, the biggest bauxite mining operation globally.
Guinea, the third-largest producer, is endowed with more than seven billion tonnes of bauxite reserves, more than any other country. Additionally, Guinea is the top exporter of bauxite globally, with 76% of its bauxite exports going to China.
After bauxite is out of the ground, it is sent to refineries across the globe to make alumina, marking the second stage of the production process.
Stage 2: Alumina production
In the 1890s, Austrian chemist Carl Josef Bayer invented a revolutionary process for extracting alumina from bauxite. Today—over 100 years later—some 90% of alumina refineries still use the Bayer process to refine bauxite.
Here are the four key steps in the Bayer process:
- Digestion:
Bauxite is mixed with sodium hydroxide and heated under pressure. At this stage, the sodium hydroxide selectively dissolves aluminum oxide from the bauxite, leaving behind other minerals as impurities. - Filtration:
Impurities are separated and filtered from the solution, forming a residue known as red mud. After discarding the mud, aluminum oxide is converted into sodium aluminate. - Precipitation:
The sodium aluminate solution is cooled and precipitated into a solid, crystallized form of aluminum hydroxide. - Calcination:
The aluminum hydroxide crystals are washed and heated in calciners to form pure aluminum oxide—a sandy white material known as alumina.
The impurities or red mud left behind in the alumina production process is a major environmental concern. In fact, for every tonne of alumina, refineries produce 1.2 tonnes of red mud, and there are over three billion tonnes of it stored in the world today.
China, the second-largest producer and largest importer of bauxite, supplies more than half of the world’s alumina.
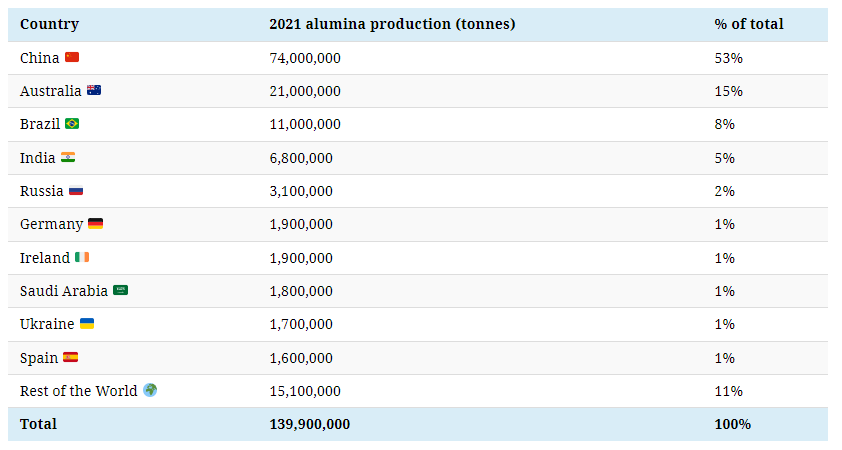
Several major producers of bauxite, including Australia, Brazil, and India, are among the largest alumina producers, although none come close to China.
Alumina has applications in multiple industries, including plastics, cosmetics, and chemical production. But of course, the majority of it is shipped to smelters to make aluminum.
Stage 3: Aluminum production
Alumina is converted into aluminum through electrolytic reduction. Besides alumina itself, another mineral called cryolite is key to the process, along with loads of electricity. Here’s a simplified overview of how aluminum smelting works:
- In aluminum smelter facilities, hundreds of electrolytic reduction cells are filled up with molten cryolite.
- Alumina (composed of two aluminum atoms and three oxygen atoms) is then dumped into these cells, and a strong electric current breaks the chemical bond between aluminum and oxygen atoms.
- The electrolysis results in pure liquid aluminum settling at the bottom of the cell, which is then purified and cast into its various shapes and sizes.
China dominates global aluminum production and is also the largest consumer. Its neighbor India is the second-largest producer, making only a tenth of China’s output.
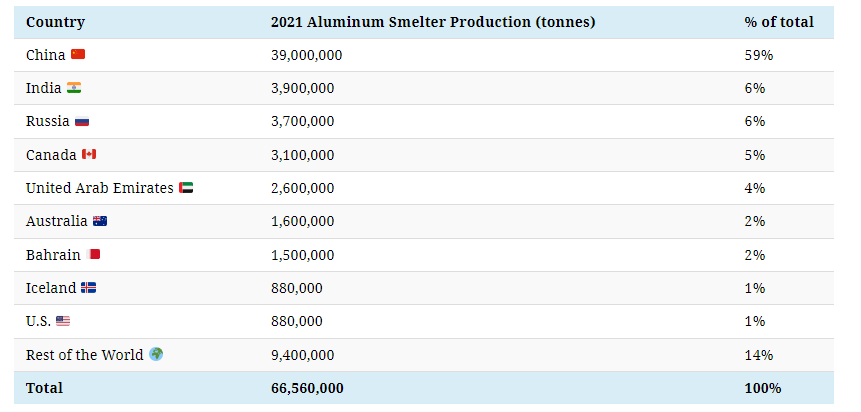
As is the case for alumina production, some of the countries that produce bauxite and alumina also produce aluminum, such as India, Australia, and Russia.
Roughly a quarter of annually produced aluminum is used by the construction industry. Another 23% goes into vehicle frames, wires, wheels, and other parts of the transportation industry. Aluminum foil, cans, and packaging also make up another major end-use with a 17% consumption share.
Aluminum’s widespread applications have made it one of the most valuable metal markets. In 2021, the global aluminum market was valued at around $245.7 billion, and as consumption grows, it’s projected to nearly double in size to $498.5 billion by 2030.
(This article first appeared in the Visual Capitalist Elements)
More News
China’s mining investment under Belt and Road Initiative sets new record – report
China's overseas mining investment under its Belt and Road Initiative hit another peak last year at $21.4 billion.
March 29, 2025 | 10:26 pm
Column: Europe’s future metals strategy hindered by current crisis
Chinese over-capacity and high energy prices have accelerated the long-term decline of European steel and aluminum production.
March 29, 2025 | 02:25 pm
{{ commodity.name }}
{{ post.title }}
{{ post.excerpt }}
{{ post.date }}







Comments