
On Tuesday, the International Energy Agency published a whopper of a report: a detailed pathway to net-zero emissions in the global energy system.
In their write-up, my colleagues Akshat Rathi and Eric Roston described the IEA as “historically staid,” and they’re not wrong.
The IEA was born of an oil crisis, and its long-term mandate has been the security of the energy supply, i.e. of enough fossil fuel to run the power, transport, and industrial processes of developed economies.
I do not think I risk overstating the significance of its decision to explore what net zero might look like.
It’s a redefinition of a guiding principle for the global energy system—from securing adequate supply to minimizing, or even zeroing out, the impacts of demand.
As frequent readers of Bloomberg Green already know well:
Science gets in the way of net-zero in some areas (e.g. cement production, which involves a chemical reaction that creates carbon dioxide), but in many, many others, ways forward do exist. Take aluminum production. Recent research from my BloombergNEF colleagues finds that not only are there pathways to zero-carbon aluminum, but those pathways can also be cheaper than sticking with fossil fuels.
Aluminum is one of the world’s most ubiquitous metals, used in everything from consumer goods to electronics to infrastructure. Producing it is energy-intensive, and at the moment, more than two-thirds of its energy consumption comes from coal and natural gas. Aluminum is responsible for about 4% of industrial emissions and 1% of all global emissions.
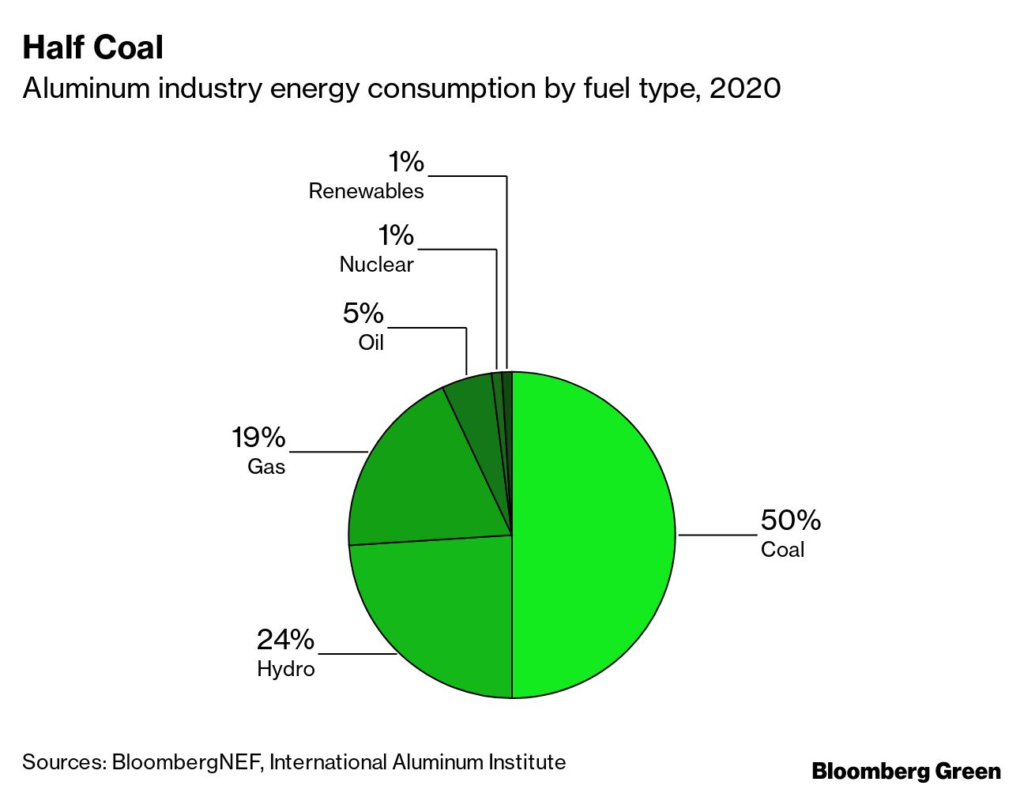
Making aluminum requires massive amounts of energy to convert aluminum oxide, or alumina, into the metal itself. That electricity consumption often results in high emissions, particularly when the electricity used is coal-fired. But because smelting is an entirely electric process, renewably generated electrons work just as well as coal-fired ones, provided they are available 24/7 to ensure stable supply. The only difference is cost—but there, renewables are often the lowest-cost option.
The other major source of emissions from smelting is the chemical process itself. As the refined alumina is zapped with a huge jolts of electricity conducted through carbon anodes, separating out oxygen atoms to create the final product, they also react with the oxygen to produce CO₂. The way to zero out emissions in aluminum production is, therefore, pretty clear: Find a way to prevent the anodes from reacting with oxygen and creating CO₂.
As Bloomberg’s Joe Deaux covered last month, there’s progress being made on that front by an interested (and interesting!) group of investors: Alcoa, Rio Tinto, Apple, the government of Canada, and the provincial government of Quebec. Their process, still in development, uses inert anodes—which don’t produce CO₂—and zero-carbon power to drive emissions to zero.
BNEF ran the numbers, and the production costs with this method could be lower than with traditional methods—and significantly lower than with processes that use carbon offsets to cancel out their CO₂ emissions (if those offsets even work).
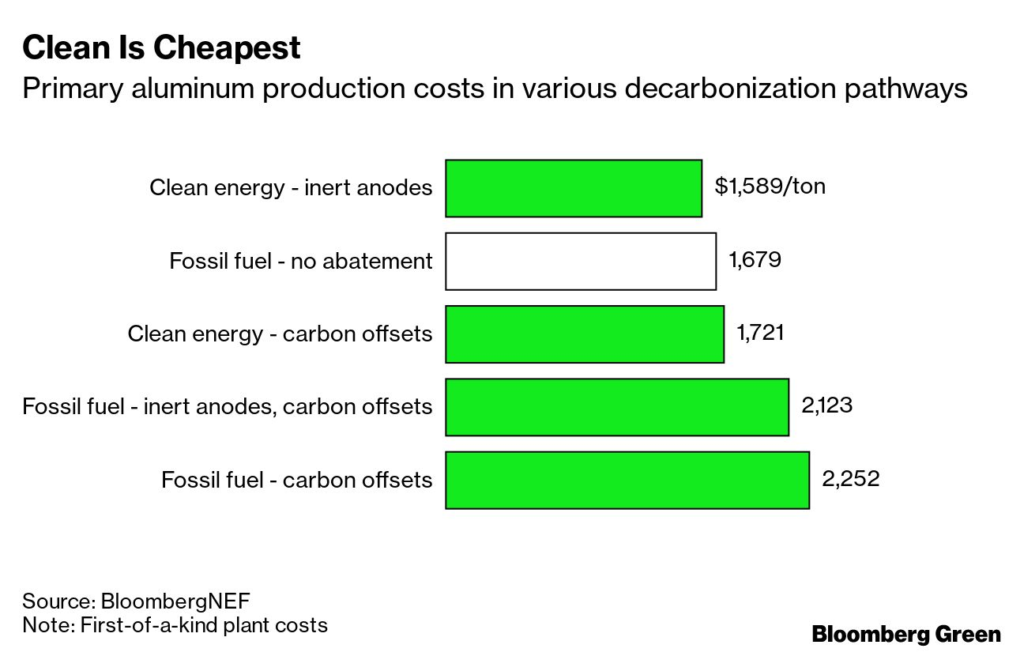
It’s easy to look at this aluminum production process and see a niche application, paid for by cashed-up corporations with reputations at stake, that would take decades to change the sector’s emissions trajectory. Skepticism is always warranted, and technology developers should welcome it.
But I believe it’s also worth looking at in a more expansive way.
Innovation in aluminum production will hopefully inspire similar innovation in equally big, equally tricky sectors. Zero emissions and a superior price point are hard to argue with, and if other industrial processes can pursue something similar, they should. Plus, aluminum’s own history is one of novelty. In the mid-19th century, aluminum was so rare and expensive to produce that Napoleon III served his state dinners on aluminum plates, while “rank-and-file guests” got gold or silver. It was still so precious by the early 1880s that aluminum was chosen for the metal cap on the Washington Monument.
Aluminum’s history is one of increasing efficiencies and new processes. Its next wave of innovations may bring it to zero emissions, and in the process help the world along a net-zero path by mid-century, too.
(By Nathaniel Bullard)
Comments