Acid Rock Drainage: The Problem with Rates
The concept of rates lies at the center of mining. As an example, the size of a mine is generally captured by identifying how many tons of ore is processed per day; which is a rate. Similarly, one the principal environmental challenges associated with hard rock mining is acid rock drainage (ARD), which is caused by the oxidation of sulfide minerals (e.g., pyrite). The mining process tends to expose and concentrate sulfide-bearing rock either during placement of waste rock or in the milling process and generation of tailing.
However, the occurrence of ARD is directly a consequence of another kind of rate; that is, the relative rate of sulfide oxidation versus the rate of acid neutralization. There are two types of acid neutralization reactions in the presence of acid produced by sulfide oxidation: (1) carbonate mineral dissolution and (2) aluminosilicate mineral dissolution. While we all learned in high school chemistry about balanced reactions, the missing concept from balanced, or equilibrium, reactions is that some chemical reactions are limited in the rate of reaction. As an example, consider the oxidation of pyrite (FeS2) in the presence of calcite (CaCO3) (a common carbonate mineral):
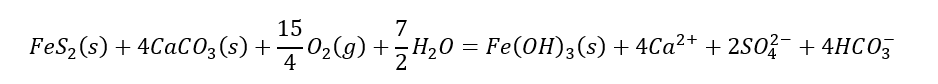
Rather than bogging down in details, think of the chemical reaction as a teeter-totter that is balanced at the equal (=) sign. The reaction says 4 parts of calcite are needed to neutralize the acidity released by oxidation of pyrite. If in an ore deposit (including waste rock) there is much more calcite that pyrite, the potential for development of acid rock drainage is low. While some mines are developed in areas with a lot of carbonate (e.g., Antamina in Peru), most mines are developed in areas where the predominant rock (e.g., granite or monzonite) is composed of aluminosilicate minerals. In this case the above equation would substitute a different mineral for calcite, say potassium feldspar:
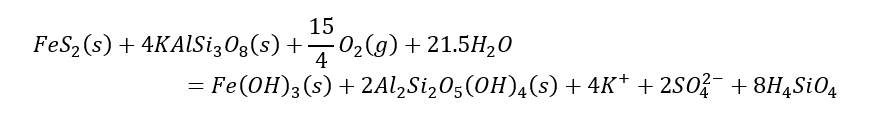
The point of the two chemical reactions listed is not to demonstrate prowess in writing balanced chemical reactions, but to demonstrate that when/if these reactions occur as written, there is no acidity (mainly in the form of H+ [or pH]) in either. That is, oxidation of pyrite by oxygen (think exposure to the atmosphere) is completely neutralized by dissolving either calcite or an aluminosilicate mineral(s).
In order of relative abundance of these chemically reactive minerals in mine rocks, aluminosilicate bearing rocks are generally in highest abundance, followed by calcite, and finally pyrite (again barring those exceptions where the ore is carbonate hosted). So why is it that ARD forms if the two equations above are true? Well, the chemical equations, again, represent equilibrium conditions; whereas in the real world (i.e., mining settings) the relative rates of the reactions (pyrite oxidation, calcite dissolution, aluminosilicate dissolution) determine the actual chemical conditions.

Acid rock drainage in a holding pond prior to treatment
Turns out there are limitations affecting the rates of mineral-specific chemical reactions. In general terms, the oxidation of pyrite is fast under the right conditions, and the rate can be further accelerated by microbial activity. For calcite, think of an antacid tablet in a glass with vinegar. The tablet begins to react immediately on contact with the vinegar, which is what happens when acidity released by the oxidation of pyrite contacts calcite.
So, the relative rates of reactions between pyrite and calcite are comparable. As for aluminosilicate minerals, there are significant limitations to the dissolution reaction due to issues in overcoming energy barriers in the detailed steps of the chemical reaction. Thus, if the only chemical reaction to offset the effects of pyrite oxidation is the dissolution of aluminosilicate minerals, ARD will more than likely form.
So, even though the mass of aluminosilicate minerals is commonly much, much more than the mass of pyrite, in the absence of calcite (or other reactive carbonate minerals), ARD will likely form in the mined rock. Much research and engineering/scientific consideration is on-going to find methods to limit the rate of pyrite oxidation and/or eliminate the reaction completely. That, however, is a topic for another future blog.

Jim Finley, Ph.D. is a Geochemist with over 20 years of experience in the application of geochemical and hydrological principles to address water quality and water management issues in environments associated with mining. He has expertise in the following technical areas: aqueous geochemistry, geochemical modeling, isotope geochemistry and hydrology, trace metal chemistry, watershed hydrology, and dynamic systems modeling.

Comments